The concept of RNA interference (RNAi) was identified in the 1980s. It is based on the selective silencing of specific sequences of mRNA, thereby, inhibiting the ability to translate into disease causing proteins.
Roots Analysis has done a detailed study on RNAi Therapeutics Market (2nd Edition), 2019 - 2030: Focus on siRNA, miRNA, shRNA and DNA, covering key aspects of the industry’s evolution and identifying potential future growth opportunities.
Key Market Insights
- Over 32,000 patents related to RNAi therapeutics, indicating the heightened pace of research, have been filed / granted in the last several years
- Presently, there is only one approved drug and over 150 product candidates, which are being evaluated for the treatment of a variety of disease indications, based on the RNAi principle
- In order to achieve a competitive edge, the drug developers are increasingly focusing on developing the robust pipeline molecules across different therapeutic areas
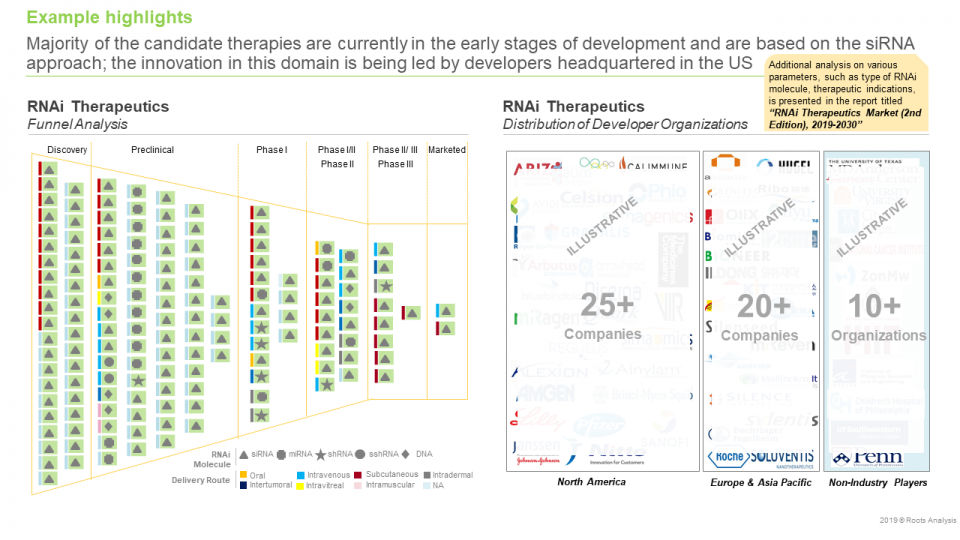
- Majority of the candidate therapies are currently in the early stages of development and are based on the siRNA approach; the innovation in this domain is being led by developers headquartered in the US
- Several trials evaluating various RNAi drug candidates against a wide range of therapeutic indications have been registered in the recent past
- Investors, having realized the opportunity within this emerging segment of the pharmaceutical industry, have invested over USD 5 billion in capital across 65 instances, in the period between 2014 and 2019
- The increasing interest in this field is reflected in recent partnership activity; majority of deals inked were R&D and licensing agreements, featuring the participation of both international and indigenous stakeholders
- In order to keep patients and healthcare professionals informed and aware of the developments in this field of medicine, companies are deploying diverse promotional strategies for their respective products
- Prevalent trends indicate that the market for RNAi therapeutics is poised to grow significantly as multiple late stage molecules are commercialized in the near future, for the treatment of different clinical conditions
TABLE OF CONTENTS
- PREFACE
- Scope of the Report
- Research Methodology
- Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
- Chapter Overview
- Historical Trends
- Discovery of RNAi
- RNAi Therapy Development Efforts
- Mechanism of RNAi
- Components of RNAi
- Cellular Mechanism
- Types of RNAi Molecules
- siRNA
- miRNA
- shRNA
- Applications of RNAi
- Advantages and Disadvantages of RNAi
- Regulatory Guidelines
- Future Perspectives
- COMPETITIVE LANDSCAPE
- Chapter Overview
- Marketed and Development Pipeline
- Analysis by Type of RNAi Molecule
- Analysis by Phase of Development
- Analysis by Type of Target Gene
- Analysis by Therapeutic Area
- Analysis by Route of Administration
- Analysis by Special Drug Designation
- Key Players
- Developer Landscape
- Analysis by Year of Establishment
- Analysis by Company Size
- Analysis by Location of Headquarters
- COMPANY COMPETITIVENESS ANALYSIS
- Chapter Overview
- Methodology
- Assumptions and Key Parameters
- Competitiveness Analysis
- LATE STAGE RNAi THERAPEUTICS
- Chapter Overview
- Onpattro®
- Drug Overview
- Technology Overview
- Current Development Status
- Recent Clinical Trial Results
- ARO-AAT
- Drug Overview
- Technology Overview
- Current Development Status
- Recent Clinical Trial Results
- Fitusiran
- Drug Overview
- Technology Overview
- Current Development Status
- Recent Clinical Trial Results
- Givosiran
- Drug Overview
- Technology Overview
- Current Development Status
- Recent Clinical Trial Results
- Inclisiran
- Drug Overview
- Technology Overview
- Current Development Status
- Recent Clinical Trial Results
- Lumasiran
- Drug Overview
- Technology Overview
- Current Development Status
- Recent Clinical Trial Results
- QPI-1002
- Drug Overview
- Technology Overview
- Current Development Status
- Recent Clinical Trial Results
- SYL 1001
- Drug Overview
- Technology Overview
- Current Development Status
- Recent Clinical Trial Results
- Vigil-EWS
- Drug Overview
- Technology Overview
- Current Development Status
- Recent Clinical Trial Results
- Vutrisiran
- Drug Overview
- Technology Overview
- Current Development Status
- Recent Clinical Trial Results
For more information, please click on the following link:
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Gaurav.Chaudhary@rootsanalysis.com