Biosimilar is also called as subsequent entry biologics or follow-on biologics. Adalimumab is a medicine used to treat psoriatic arthritis, rheumatoid arthritis, and various other diseases. Adalimumab biosimilar is equally effective and safe in reducing inflammation as Humira.
Global Adalimumab Biosimilar Market was valued US$ XX Mn in 2019 and is expected to grow US$ XX Mn by 2027, at a CAGR of XX% during the forecast period.
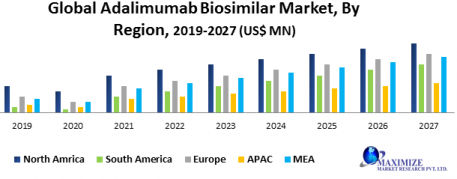
Global Adalimumab Biosimilar Market1
The report study has analyzed the revenue impact of the COVID-19 pandemic on the sales revenue of market leaders, market followers, and market disrupters in the report, and the same is reflected in our analysis.
Biosimilar is also called as subsequent entry biologics or follow-on biologics. Adalimumab is a medicine used to treat psoriatic arthritis, rheumatoid arthritis, and various other diseases. Adalimumab biosimilar is equally effective and safe in reducing inflammation as Humira.
The global market for adalimumab biosimilar is expected to grow the market from 2019 to 2027 thanks to growing incidence of arthritis among the worldwide population. The major trend in the market is high investment in the manufacturing and research of the new adalimumab biosimilars. However, the global adalimumab biosimilar market is hampered by the shortage of skillful specialists to operate. Also, the factor like the high sensitivity towards temperature, absence of efficacy and safety, high manufacturing cost related to generic drugs are expected to limit the market growth during the 2019-2027.
By distribution channel, the retail pharmacy is expected to grow at the highest XX% CAGR during forecast period because of the rapidly increasing adoption of retail pharmacy portals to buy the drugs. Similarly, the report covers the segments in the adalimumab biosimilar market such as product, and distribution channel.
Region-wise, North America is expected to hold the largest market XX% share of the adalimumab biosimilars market during the forecast period, thanks to the increasing occurrence of rheumatoid arthritis with the rise in the aged population. However, Europe is the second-largest market, because of a rise in the approvals of the new biosimilars, a rise in the aged population, high investment in the research and manufacturing of the new adalimumab biosimilars. From 2018, five new adalimumab biosimilars names as ABP501 (Amgevita), SB5 (Imraldi), GP2017 (Hyrimoz), FKB327 (Hulio), MSB11022 (Idacio) have been permitted in Europe. ABP501 was the first European Commission permitted adalimumab biosimilar. It is sold by Amgen under the brand name AMGEVITA. These new biosimilars are expected to drive the growth of the global adalimumab biosimilars market in the forecast period.
Companies are involved in adopting sustainable strategies to gain competitive edges such as new product launch, product up gradation and collaborative agreements. For example, in Oct 2018, Amgen Company launched AMGEVITA a biosimilar of adalimumab.
The objective of the report is to present a comprehensive analysis of the Global Adalimumab Biosimilar Market including all the stakeholders of the industry. The past and current status of the industry with forecasted market size and trends are presented in the report with the analysis of complicated data in simple language. The report covers all the aspects of the industry with a dedicated study of key players that includes market leaders, followers, and new entrants. PORTER, SVOR, PESTEL analysis with the potential impact of micro-economic factors of the market has been presented in the report. External as well as internal factors that are supposed to affect the business positively or negatively have been analyzed, which will give a clear futuristic view of the industry to the decision-makers.
The report also helps in understanding Global Adalimumab Biosimilar Market dynamics, structure by analyzing the market segments and projects the Global Adalimumab Biosimilar Market size. Clear representation of competitive analysis of key players By Product, price, financial position, product portfolio, growth strategies, and regional presence in the Global Adalimumab Biosimilar Market make the report investor’s guide.
Scope of the Global Adalimumab Biosimilar Market
Global Adalimumab Biosimilar Market, By Product
• Exemptia
• Adalirel
• Cipleumab
• Others
Global Adalimumab Biosimilar Market, By Distribution Channel
• Hospitals Pharmacies
• Retail Pharmacies
• Others
Global Adalimumab Biosimilar Market, By Region
• North America
• Europe
• Asia Pacific
• Middle East & Africa
• South America
Key players operating in the Global Adalimumab Biosimilar Market
• Alfred E. Tiefenbacher (GmbH & Co. KG)
• Amgen Inc.
• Boehringer Ingelheim International GmbH
• Glenmark
• Zydus Cadila
• Torrent Pharmaceuticals Ltd.
• Reliance Life Sciences
• Emcure Pharmaceuticals Ltd
• Cipla Inc.
• Hetero
• Others
For More Information Visit @: Maximize Market Research Company
This Report Is Submitted By : Maximize Market Research Company
Customization of the report:
Maximize Market Research provides free personalized of reports as per your demand. This report can be personalized to meet your requirements. Get in touch with us and our sales team will guarantee provide you to get a report that suits your necessities.
About Maximize Market Research:
Maximize Market Research provides B2B and B2C research on 20,000 high growth emerging opportunities & technologies as well as threats to the companies across the Healthcare, Pharmaceuticals, Electronics & Communications, Internet of Things, Food and Beverages, Aerospace and Defense and other manufacturing sectors.
Contact info:
Name: Lumawant Godage
Organization Address: MAXIMIZE MARKET RESEARCH PVT. LTD.
Email: sales@maximizemarketresearch.com
Address: Pune, Maharashtra 411051, India.
Contact: +919607195908