The “RNAi Therapeutics Market (2nd Edition), 2019-2030: Focus On siRNA, miRNA, shRNA and DNA” report features an extensive study of the current market landscape and future opportunities associated with RNAi therapeutics. The study also features a detailed analysis of key drivers and trends within this evolving market.
The number and geographical distribution of clinical trials are important indicators of both the therapeutic viability and future potential of innovative pharmacological interventions. It is obvious that pharmaceutical / biopharmaceutical companies would invest in conducting extensive clinical studies for only those drug / therapy classes that are likely to translate into commercial success. Moreover, the geographical distribution directly indicates the various markets that are open to receiving / adopting the intervention(s) under investigation. As more product candidates get approved by regulatory authorities across the globe, the number of clinical trials across different global regions is anticipated to increase.
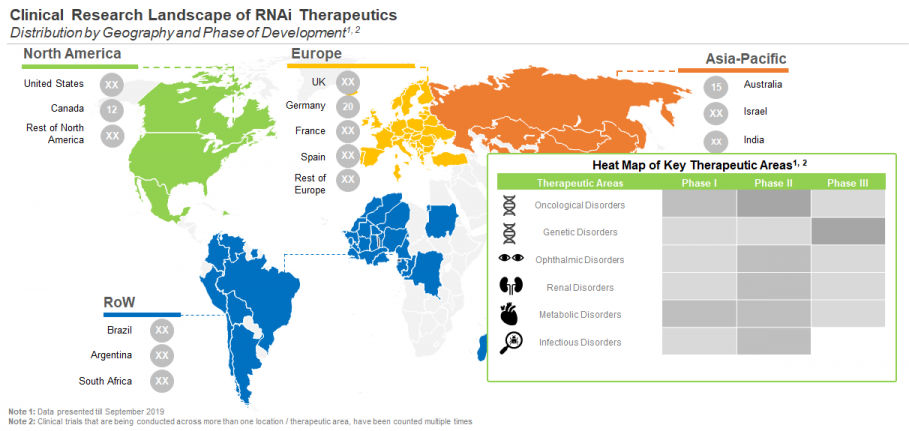
Close to 20% of the clinical trials for RNAi therapeutics have been / are being conducted for genetic disorders and oncology. It is followed by metabolic disorders (13%), ophthalmic disorders, infectious disorders and cardiovascular disorders each 8%. It is important to mention that majority of RNAi therapeutics being evaluated for infectious diseases are targeting hepatitis B and hepatitis C. It is worth mentioning that, Onpattro, the first marketed RNAi therapeutic (Alnylam Pharmaceuticals), is intended for the treatment of hTTR amyloidosis, which is a genetic disorder
Majority of the RNAi therapeutics being evaluated in phase I (22%) and phase II (26%) trials target oncological indications. On the other hand, most (41%) of the molecules being evaluated in phase III trial target genetic disorders. It is worth highlighting that 40% of phase III trials in Europe are evaluating RNAi therapeutics for the treatment of genetic disorders.
Most of the completed / ongoing / planned trials for RNAi therapeutics are centered in Europe. Further, within this region, UK (27) emerged as the most prominent center for the clinical trials related to RNAi therapeutics. Within North America, the maximum number of trials (70) were reported to be / have been conducted in US. In Asia, till September 2019, the maximum number of trials were registered in Australia (15), followed by India (8).
Maximum number of patients were enrolled in studies centered in Europe. Further, within Europe, most of the patients were enrolled in UK (16,508) for studies of RNAi therapeutics. In North America, the maximum number of patients were enrolled for RNAi therapeutics-based studies in the US (9,249). Within the Asia region, most of the patients were enrolled in studies based in Australia (387).
The below table highlights all the late-stage clinical trials (phase II/III phase III) for RNAi therapeutics that are expected to end (primary completion) in 2019 and 2020.
| Type of RNAi Molecule | Drug Name | Developer | NCT Number | Phase | Target Patients Enrolled | Primary Completion Date |
| siRNA | Givosiran (ALN-AS1) | Alnylam Pharmaceuticals | NCT03338816 | Phase III | 94 | Jan-19 |
| Fitusiran (ALN-AT3) | Alnylam Pharmaceuticals | NCT03417245 | Phase III | 120 | Apr-20 | |
| NCT03417102 | Phase III | 54 | Feb-20 | |||
| Inclisiran (ALN-PCSsc) | Alnylam Pharmaceuticals | NCT03399370 | Phase III | 1,561 | Oct-19 | |
| NCT03400800 | Phase III | 1,617 | Oct-19 | |||
| NCT03397121 | Phase III | 482 | Sep-19 | |||
| Lumasiran (ALN-GO1) | Alnylam Pharmaceuticals | NCT03681184 | Phase III | 30 | Dec-19 | |
| NCT03905694 | Phase III | 6 | Mar-20 |
As can be observed from the table, a total of eight phase III clinical trials evaluating four drug candidates, using siRNA molecules, for the treatment of different indications are expected to complete in the coming two years. On the other hand, drugs based on miRNA, namely Cobomarsen (MRG-106) and Remlarsen (MRG-201), which are being developed by miRagen Therapeutics, are currently in phase II clinical trials likely to be completed by December 2021 and April 2020, respectively. In addition, vigil, a shRNA-based drug being developed by Gradalis, is currently in phase III, expected to be completed by July 2022. Further, BB-401, a DNA based drug being developed by Benitec Biopharma, is currently being evaluated in a phase II clinical trial which is likely to reach completion by April 2019.
Scope of the Report
The “RNAi Therapeutics Market (2nd Edition), 2019-2030: Focus On siRNA, miRNA, shRNA and DNA” report features an extensive study of the current market landscape and future opportunities associated with RNAi therapeutics. The study also features a detailed analysis of key drivers and trends within this evolving market. Amongst other elements, the report includes:
- A detailed review of the overall landscape of companies developing RNAi therapeutics, including information on phase of development (marketed, clinical, and preclinical / discovery stage) of pipeline candidates, target disease indication(s), key therapeutic areas (oncological disorders, infectious diseases, genetic disorders, ophthalmic diseases, respiratory disorders, hepatic disorders, metabolic disorders, cardiovascular disorders, dermatological disorders, and others), type of RNAi molecule (siRNA, miRNA, shRNA, sshRNA and DNA), target genes, type of delivery system used, route of administration and special drug designations (if any).
- A competitiveness analysis of key players engaged in this domain, evaluating their respective product portfolios, type of RNAi molecule, target therapeutic areas, company size and year of establishment.
- An analysis of completed, ongoing and planned clinical studies for different types of RNAi molecules. The trials were analyzed on the basis of various relevant parameters, such as registration year, current status, phase of development, type of RNAi molecule, regional distribution of clinical trials and enrolled patient population.
- An in-depth analysis of the various patents that have been filed / granted related to RNAi therapeutics, since 2014. The analysis also highlights the key parameters associated with the patents, including information on patent type (granted patents, patent applications and others), publication year, regional applicability, CPC symbols, emerging focus areas, leading industry / non-industry players (in terms of the number of patents filed / granted), and patent valuation.
- An analysis of the various partnerships pertaining to RNAi therapeutics, which have been established till August 2019, based on various parameters, such as the type of partnership, year of partnership, target disease indications, therapeutic area, type of RNAi molecule, financial details (wherever applicable), focus area of collaboration and most active players.
- An analysis of the investments made at various stages of development in companies engaged in this domain, between 2014-2019, including seed financing, venture capital financing, IPOs, secondary offerings, debt financing, grants and other offerings.
- An analysis of the key promotional strategies that have been adopted by developers of marketed oligonucleotide therapeutics, namely Defitelio®, Exondys® and Onpattro®.
- A review of emerging technology platforms and delivery systems that are being used for targeted therapeutic delivery, featuring detailed profiles of technologies.
- Detailed profiles of drug candidates that are in the advanced stages of development (phase II/III and above), including information on their current development status, mechanism of action, route of administration, affiliated delivery technology, dosage, recent clinical trial results along with information on their respective developers.
- An elaborate discussion on the use of miRNA as a potential biomarker, along with a list of diagnostic kits that are either available in the market, or likely to be approved in the foreseen future.