Prefilled syringes have been in market for over two decades now and have witness significant growth in terms of both adoption and revenues. The biopharmaceutical industry, given the fact that biologics are predominantly administered via the parenteral route, has been one of the key drivers of this market.
Prefilled syringes have been in market for over two decades now and have witness significant growth in terms of both adoption and revenues. The biopharmaceutical industry, given the fact that biologics are predominantly administered via the parenteral route, has been one of the key drivers of this market. A brief discussion on further growth opportunities and the anticipated future of the prefilled syringes market is provided below:
- Increased focus on improving drug device compatibility: One of the major challenges for the manufacturers in this industry is the negative interactions of prefilled syringe barrels with the drug. This issue is of great concern for the developers as it affects the stability of the drug. To overcome this, manufacturers need to eliminate the interaction between drugs and packaging materials. As syringes are being used more and more today, hence regulatory bodies, companies and customers will scrutinize and look carefully at each and every aspect of needles and the material that are being used to develop a prefilled syringe.
- Increase in investments on advanced syringe development and manufacturing technologies: More complex technology is required to keep up with the pace of pharmaceutical and biotechnology developments this calls for more investments in the manufacturing and development of syringes. The number of injectable products available are increasing in the market and this trend is likely to continue in the foreseeable future. Further, an increase in flexibility is also being requested by suppliers. This changing trends in the market indicates that the technology also needs to be developed as per these changes. Additionally, if a large number of new drugs are delivered through prefilled syringes then the penetration rate and uptake of prefilled syringes by the end users is likely to increase, as the end users are likely to get used to this format of drug delivery. Hence, in order to keep up with this growing demand and to maintain the competitiveness in this market domain manufacturers will need to investment more for the improvement of manufacturing technology and process.
- Efforts to develop safety solutions for prefilled syringes: Safety measures to eliminate needle stick injuries are the biggest concern in the healthcare industry. The safety systems are very costly and hence, the manufacturing costs of syringes is increasing. To address this issue, the safety system developers are continuously researching to develop low cost alternatives. In the current market, the percent of prefilled syringes with safety devices is low however, this trend is likely to change in the forthcoming years as the healthcare industry is likely to increase the focus on needlestick safety aspects.
- Growing trend of individualization: As the demand for prefilled syringes is increasing, manufacturers are beginning to differentiate themselves by focusing on the development of individualized solutions for various purposes and applications. In other words, the current focus among syringe developers is on offering novel features, such as visual distinction, enhanced precision, ergonomic design for easy handling and advanced safety provisions. This is how brands are working towards becoming more recognizable in the already saturated and highly competitive market.
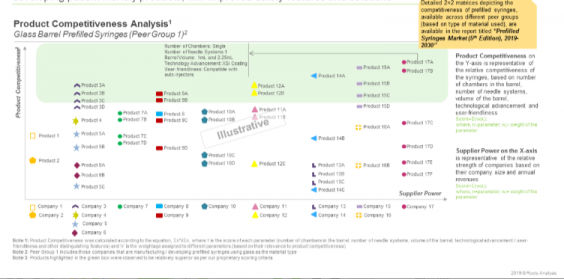
Owing to these aforementioned opportunity areas, several prefilled syringe manufacturers are actively involved in development of prefilled syringes that are more patient friendly, have improved safety features and solutions. In our report, we have presented an analysis of the relative competitiveness (based on a number of relevant parameters) of the prefilled syringes identified during our research. Specifically, we compared around 70 such products across two peer groups, which were defined based on the barrel material used. The analytical output was represented in the form of a 2×2 matrix, with supplier power as the abscissa and product competitiveness on the ordinate axes.
The purpose of the analysis was to develop a better understanding of the current generation of prefilled syringes and their respective specifications and key features. The insights presented in this chapter are indicative of the current benchmarks / standards related to the development of such products. It is important to mention that the data used for this analysis was collated from publicly available sources. Moreover, at no point should the discussion in this chapter be construed as a recommendation that one product is better than another.