According to a study, 35 million health care coworkers are at a risk of needlestick injuries, globally. The US CDC has estimated that 600,000–1,000,000 needlestick injuries occur annually.
According to a study, 35 million health care coworkers are at a risk of needlestick injuries, globally. The US CDC has estimated that 600,000–1,000,000 needlestick injuries occur annually. Among the healthcare professionals, in 45% of the cases, nurses are harmed by the needlestick injuries, followed by technicians and doctors who get injured in 20% cases, each and maintenance workers. As per the WHO estimates, a person who gets pricked accidentally by an infected needle has 30% risk for developing HBV, 1.8% risk for HCV and 0.3% for HIV. In addition to the aforementioned diseases, there are over 60 bloodborne pathogens that can also infect a person who experiences needlestick injury. Moreover, the situation is grave in developing countries where close to 75% of needlestick injuries are not even reported.
The estimated cost of treatment of a single needlestick injury ranges from USD 500 to USD 4,000 in the US. With 236,000 cases of needlestick injuries, the economic burden in the US is estimated to be USD 118 million to USD 591 million. It is worth highlighting that this cost may increase depending upon the seriousness of the case. Further, as per the American Hospital Association, one serious case of pathogen based infectious disease can cost up to USD 1 million or even more than this depending upon the expenditures on the testing, follow up tests, lost time and disability payments.
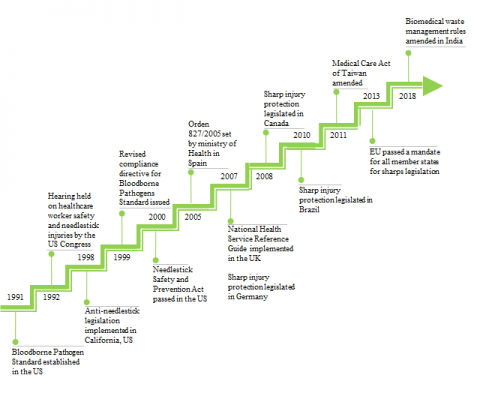
The frequency of occurrence of needlestick injuries has also garnered the interest of other governments across the world to enact laws to protect healthcare workers from such harm. In addition, various strategies to mandate the use of safety devices to prevent needlestick injuries are being adopted. International markets, such as Europe, Canada and Australia are transitioning to the use of safety products. In May 2013, this new EU Directive, 2010/32/EU, came into force in all member states of the EU. Furthermore, in India, new biomedical waste management rules were amended in 2018, as per which, it is mandatory to report needlestick injuries. As per a study conducted in 2015, the use of safety devices for needles and syringes is less in this region since there are no regulations that govern the use of such devices. Above all, the WHO has recommended the use of safety devices and instructed the governments for overall transition to the exclusive use of safety devices by 2020.
Figure given below shows the evolution of healthcare safety legislations across the various regions of the world.
In compliance with needlestick injuries prevention laws and legislations, prefilled syringes now come with safety devices to reduce the risk of such injuries. These safety devices are of two types:
- Passive: Devices that automatically shield the needle and need not be activated manually.
- Active: Devices that are required to be activated by the user once the injection process is complete.
It is now mandatory in almost all international healthcare markets to use safety-engineered devices to protect healthcare workers from risk of getting exposed to contaminated needles and prevent needlestick injuries.
Currently, most of the prefilled syringes do not have integrated safety features. Traditionally, prefilled syringe manufacturers sourced needle guard devices from elastomer components manufacturers. Examples of some of the add-on safety device manufacturers include Becton Dickinson, West Pharmaceutical, Vetter Pharma, Tip-Top, Schreiner Medipharm and Credence Medsystems. The safety devices can be passively or manually activated upon completion of injection. These ancillary devices slide an external plastic sheath over the entire prefilled syringe or have a needle retraction system that upon activation, retracts the needle back into the barrel to protect the user from needlestick. Integration with a standard and approved staked-in or luer connection syringe and syringe components reduces the incompatibility risk for the drug formulation, as well as the development effort and risk of the drug manufacturers. This approach can significantly reduce the timeline for development and regulatory approval of prefilled syringes.
On the other hand, the disadvantage of using these clip-on safety devices is that they are often much larger in size than the syringe itself increasing the overall packaging and shipping costs. The ancillary safety products can increase pharmaceutical packaging, transport and disposal volumes by up to 70%.
Manufacturers of prefilled syringes, in compliance with safety laws, are incorporating safety features in their devices. As per a study conducted by MedPro Safety Products, about 15% of the prefilled syringes in the market use add-on safety devices. The percentage of prefilled syringes with integrated safety features is even lower. The companies that market prefilled syringes with integrated safety features include Gerresheimer, MedicalChain, Ompi and Taisei Kako. MedicalChain is developing integrated syringes that use approved barrel and syringe components from leading suppliers. Therefore, this offers the advantages to the add-on safety devices in terms of reduced compatibility, development and regulatory risk.






*********nkklin@gmail.com
Pod systems and disposable vapes have become increasingly popular for their convenience and discreetness, allowing users to vape on-the-go without hassle. This trend Vaping aligns with the evolving lifestyles of consumers seeking convenience without compromising on the vaping experience.