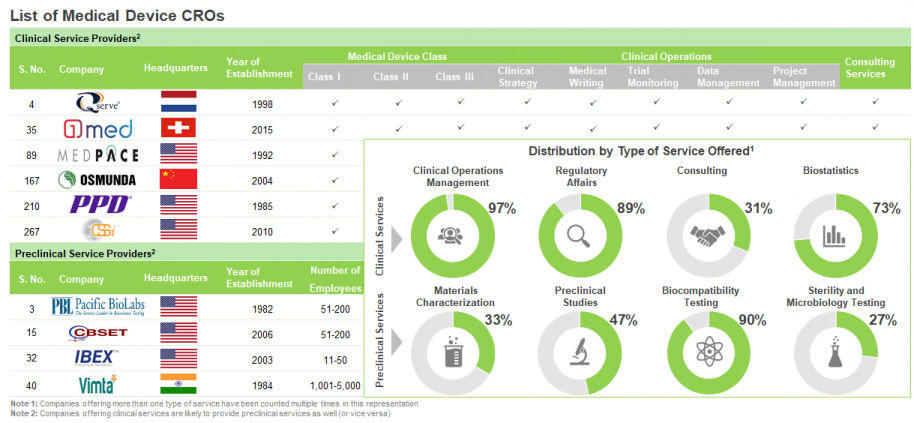
During our research, we were able to identify over 250 industry players that presently claim to offer contract services for clinical development of medical devices. In addition, there are more than 40 companies that are presently offering contract services for preclinical development of medical devices. The market is currently dominated by the presence of very small and small companies (less than 51 employees), that represent over 50% of the total players. In fact, in the past few years, the domain has witnessed the establishment of several start-ups.
In terms of geography, more than 70% of medical device CROs are based in North America and Europe. Within North America, majority of the service providers are headquartered in the US, in fact, over 50% of preclinical service providers are US-based; whereas, in Europe, most of the CROs are distributed across France, Germany, Switzerland, Spain, Italy and Sweden. Further, a significant number of such players (30%) are headquartered in Asia-Pacific and other developing countries of the world.
In terms of device class, majority of the clinical CROs (79%) offer services for all device classes. However, only a limited CROs (11%) provide services for both class II and class III devices, while close to 5% companies offer services for both class I and class II devices. In addition, close to 50% CROs claim to have elaborate clinical service portfolio, offering end-to-end solutions. Majority of such players offer assistance in clinical trial monitoring (83%) and clinical data management (83%); other popular services include (in terms of number of companies offering them) those provided for initiating clinical studies (79%), medical writing (78%), devising a clinical strategy (75%), and clinical project management (70%).
In terms of preclinical services offered, biocompatibility testing (42) is the most common type of service offered by medical device preclinical CROs, followed by services for conducting preclinical studies (23). Apart from these, various CROs also provide material characterization and analytical services (22), regulatory / certification services (21), and sterility and microbiology testing services (19). Most of the preclinical CROs that were established before 2005 and offer biocompatibility testing services are based in North America. Further, majority of players that were established after 2005 and offer the aforementioned service were observed to be headquartered in North America, and Asia-Pacific and rest of the world. It is worth noting that most of the companies providing package integrity testing services and established before 2005 are based in North America, whereas majority of such players established after 2005 are based in Asia-Pacific and rest of the world.
In addition, there are over 160 industry players that are presently offering standalone services for medical devices. Close to 60% standalone service providers were established post 2000. Examples of companies established post 2015 include (in alphabetical order) APO Plus Station, Bioreg Services, Clarivate Analytics, Consultys, dn8 collaborate, Dove Quality Solutions, IVDeology, IZiel Healthcare, MDP Solutions, QUNIQUE, Ulmer Ventures and Voler Biotech Consulting.