Medical Device CRO Market is estimated to be worth USD 15.7 billion in 2030, predicts Roots Analysis
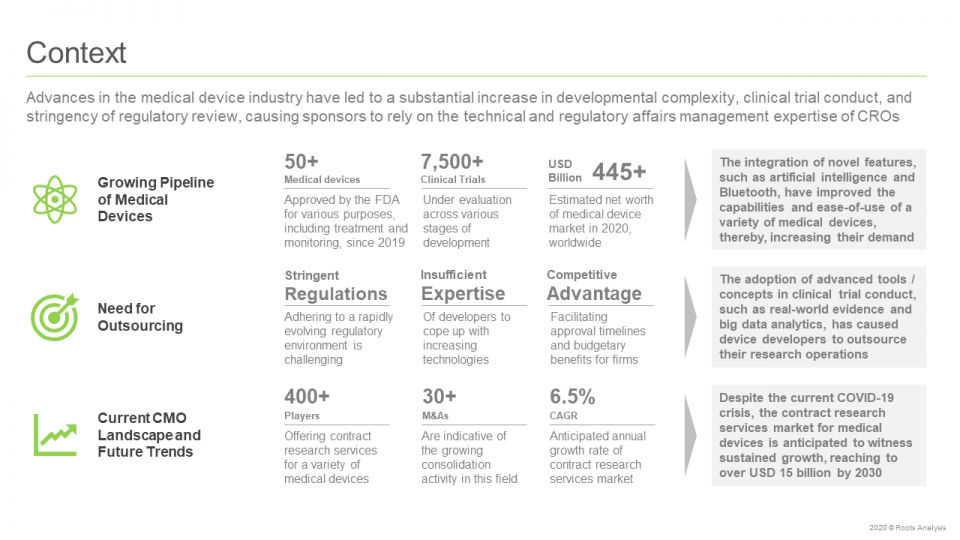
Advances in the medical device industry have led to a substantial increase in developmental complexity, clinical trial conduct, and stringency of regulatory review, causing sponsors to rely on the technical and regulatory affairs management expertise of CROs
Roots Analysis is pleased to announce the publication of its recent study, titled, “Medical Device CRO Market (2nd Edition), 2020-2030”.
The report features an extensive study of the current market landscape and future opportunities of contract research service providers focused on medical devices. The study also features an in-depth analysis, highlighting the capabilities of the various stakeholders engaged in this domain, across different regions of the globe. In addition to other elements, the study includes:
- A detailed review of the overall landscape of medical device CROs.
- An elaborate discussion on the various guidelines established by major regulatory bodies for medical device approval across North America, Europe, and Asia-Pacific and rest of the world.
- Elaborate profiles of key players that specialize in offering services for both clinical and preclinical stage development of medical devices.
- An analysis highlighting the key performance indicators used by sponsor companies to evaluate service providers engaged in this domain
- A competitive benchmarking, highlighting the key focus areas of small, mid-sized and large companies, comparing their existing capabilities within and beyond their respective peer groups.
- A detailed brand positioning analysis of leading industry players (shortlisted on the basis of strength of service portfolio).
- A detailed geographical clinical trial analysis of ongoing and planned studies related to medical devices.
- A detailed analysis of the mergers and acquisitions that have taken place in this domain during the period 2015-2020.
- A survey analysis featuring inputs solicited from various experts who are directly / indirectly involved in providing contract research services to medical device developers.
- A discussion on affiliated trends, key drivers and challenges, under a SWOT framework, which are likely to impact the industry’s evolution.
- An elaborate discussion on the future opportunities / trends for the medical device outsourcing market that are likely to influence the growth of this domain over the coming years.
A detailed market forecast, featuring analysis of the current and projected future opportunity across key market segments (listed below)
Phase of Development
- Clinical
- Preclinical
Types of Preclinical Services Offered
- Biocompatibility testing
- Sterility and microbiology testing
- Material characterization and analytical services
- Others
Types of Clinical Services Offered
- Clinical trial management
- Data management
- Regulatory affairs management
- Consulting
- Others
Device Class
- Class I medical devices
- Class II medical devices
- Class III medical devices
Target Therapeutic Area
- CNS disorders
- Cardiovascular disorders
- Oncological disorders
- Bone disorders
- Respiratory disorders
- Pain management disorders
- Ophthalmic disorders
- Psychological disorders
- Metabolic disorders
- Others
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
Transcripts of interviews held with the following senior level representatives of stakeholder companies
- Lajos Sarosi (Chief Executive Officer and Co-founder, HungaroTrial)
- Christopher Rupp (Vice President of Global Marketing and Commercial Operations, NAMSA)
- Christian Wolflehner (General Manager, CW Research & Management)
- Troy Mccall (Chief Operating Officer, CROMSOURCE)
- Nazish Urooj (Senior manager, Medical & Clinical Operations, Metrics Research)
- C. Omprakash (Technical Director and Partner, Vyomus Consulting)
- Tania Persson (Director of Business Development, A+ Science)
- Alexa Foltin-Mertgen (Business Development Manager, AtoZ-CRO)
Key companies covered in the report
- Avania (formerly known as Factory CRO)
- Charles River Laboratories
- Clinlogix
- CROMSOURCE
- CSSi LifeSciences™
- Eurofins Medical Device Testing
- genae
- IMARC Research
- IQVIA
- Medpace
- NAMSA
- Qserve Group
- Regulatory and Clinical Research Institute (now a part of Covance)
- WuXi AppTec
For more information, please click on the following link:
https://www.rootsanalysis.com/reports/view_document/medical-device-cros-market/226.html
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415